This page is not the official website. Generated by AI, does not constitute any advice. If needed, please click here to visit the corresponding official website.
Skyline Therapeutics is a fully-integrated gene therapy company dedicated to addressing unmet medical needs in rare and severe diseases through innovative research and development.

The field of gene and cell therapy is rapidly advancing, yet major unmet medical needs remain for patients with rare or severe diseases. Skyline Therapeutics strives to address these unmet needs through the research and development of novel therapies that have potential to bring life-changing benefits to patients.
We are committed to innovation, scientific excellence, and patient-centric development across our pipeline and platform technologies.
Explore Our Science →From discovery to CMC, Skyline has established an advanced adeno-associated virus (AAV) platform integrating capsid discovery, vector engineering, process development, and GMP manufacturing.

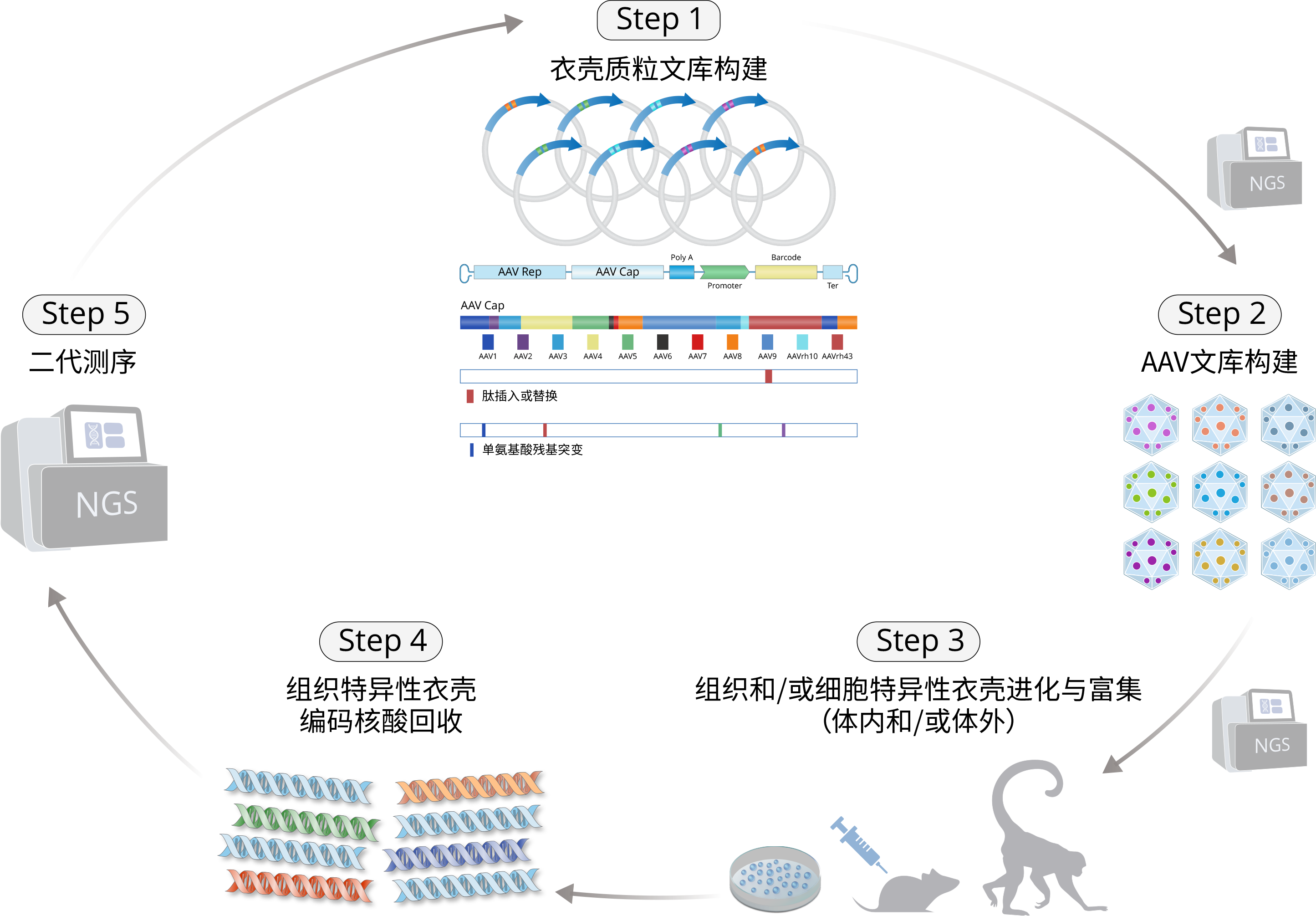
Our capDRIVE® platform enables efficient identification of novel capsids with desired tissue targeting and immune evasion properties.
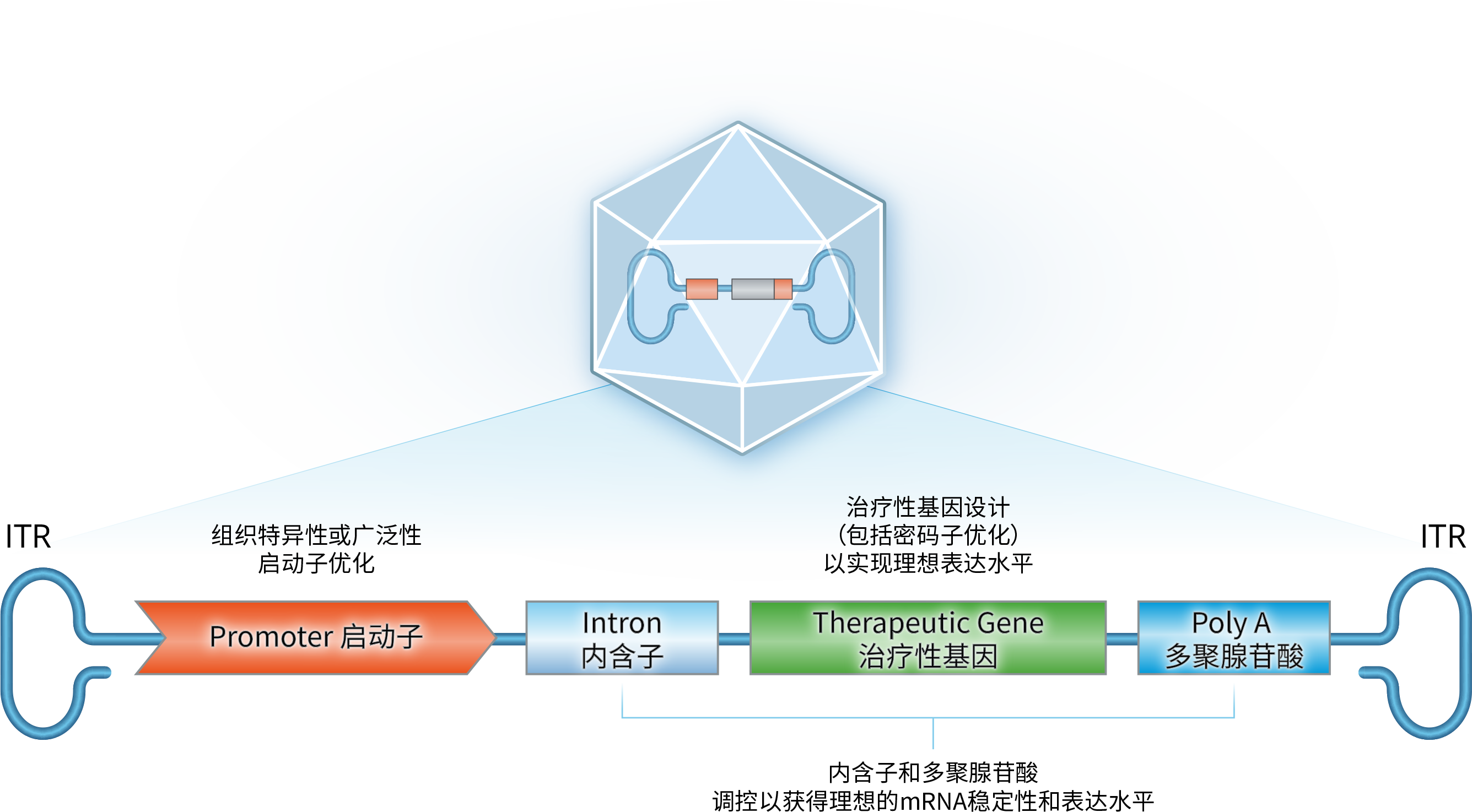
Proprietary vector engineering enhances transgene expression, stability, and safety profiles for improved therapeutic outcomes.
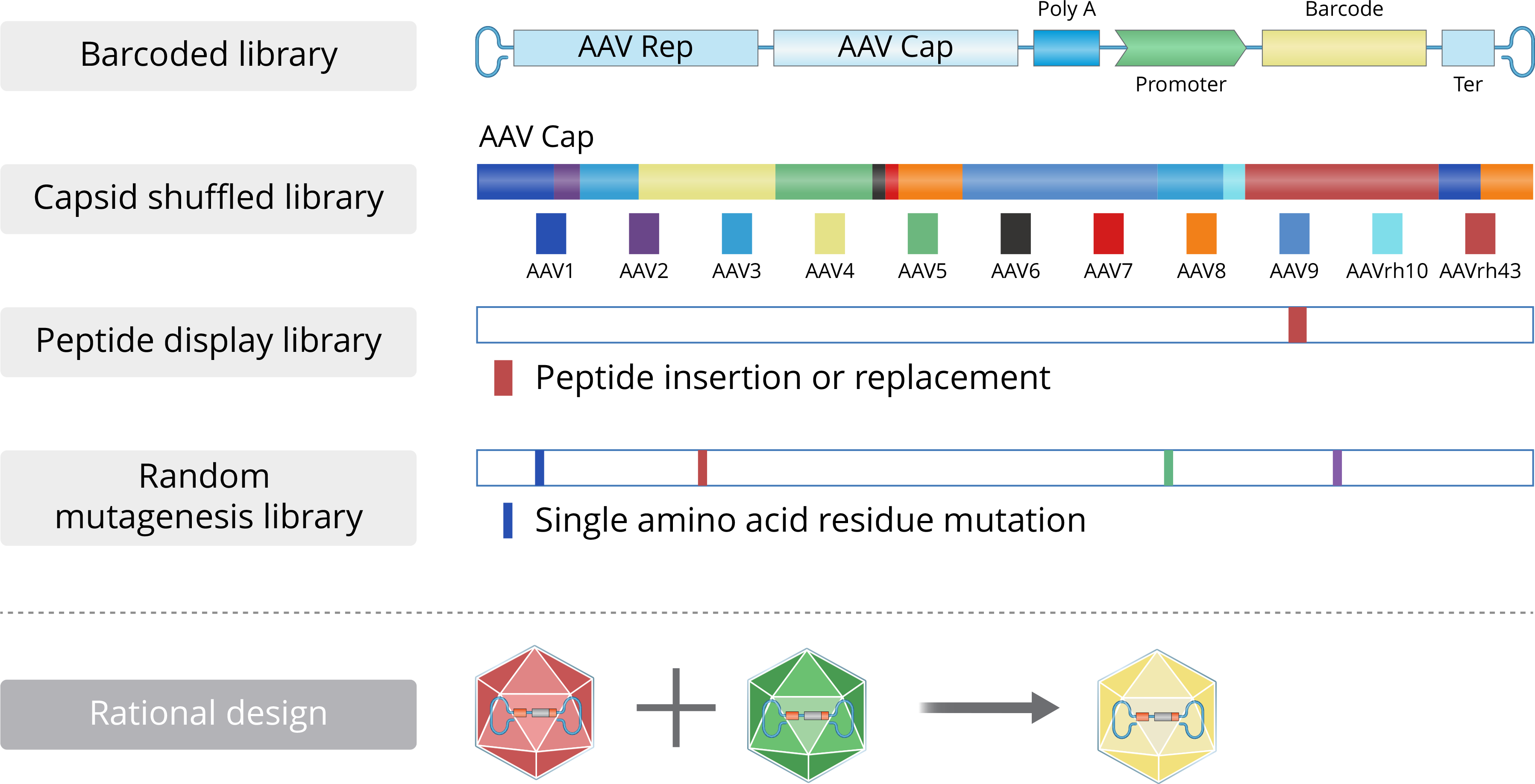
Scalable, high-yield manufacturing processes ensure consistent quality and supply for clinical and commercial needs.
Advancing a pipeline of innovative gene therapies targeting rare and severe diseases with high unmet need.

Optogenetic Gene Therapy for Retinitis Pigmentosa

AAV Gene Therapy for Neurodegenerative Disease

Gene Therapy for Rare Metabolic Disorder

Gene Therapy for Muscular Dystrophy

AAV-Based Immunomodulatory Therapy

Oncolytic AAV for Solid Tumors

Gene Therapy for Rare Lung Disease

Cardiovascular Gene Therapy
We partner with academic institutions, biotech companies, and patient advocacy groups to accelerate innovation and expand access to transformative therapies.
Skyline Therapeutics believes in the power of collaboration to drive scientific breakthroughs. Our partnerships are built on shared values of innovation, integrity, and patient focus.
We are actively seeking strategic alliances in gene therapy research, technology development, and clinical translation.

At ASGCT 2025, Skyline presented new data on SKG1108, a novel optogenetic gene therapy for retinitis pigmentosa, demonstrating promising preclinical results.
The U.S. FDA has granted Orphan Drug Designation to SKG1108 for the treatment of retinitis pigmentosa, recognizing its potential to address a rare disease with no approved therapies.
Skyline shared updates across its pipeline, including preclinical data for SKG2201 and SKG3305, highlighting the breadth of its gene therapy platform.
Reach out with general questions, job opportunity inquiries, or for partnership discussions.
2/F Building C, Jinke Center
No. 2727 Jinke Road, Zhangjiang Science City
Shanghai, China
Building 12, Medicine Port Town
Fucheng Road, Hangzhou
Zhejiang, China
1 Main Street, 13th Floor
Cambridge, MA 02142
USA
Notice for Buyers: If you are a buyer, the above information is generated by AI collection and does not constitute any advice. If necessary, please visit the corresponding official website.
For Domain Owners: If you are the domain owner and do not want to be included by fobcompany.info, please contact support@fobcompany.info via your corporate email to cancel. We will cancel your inclusion within 3 business days.
如果您是域名所有者 不想被fobcompany.info收录 请用企业邮箱联系support@fobcompany.info 取消收录 我们将在3个工作日取消您的收录
Service Request: If you are other domain and want to be included, please contact support@fobcompany.info
如果您是其他域名合作 也请联系support@fobcompany.info